16. A copper can of negligible heat capacity contains 1.0 kg of water just above the freezing point. A similar can contains 1.0 kg of water just below the boiling point. The two cans are brought into thermal contact. What is the change in entropy of the system? The specific heat capacity of water is 4180 J 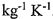 .
.
(A) +404 J/K
(B) +202 J/K
(C) +101 J/K
(D) The entropy is unchanged
(E) -202 J/K
答案:登入後查看
統計: 尚無統計資料
統計: 尚無統計資料