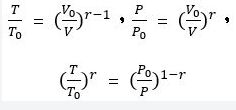8. 假設某一氣體進行斷熱壓縮(Adiabatic process),壓縮前體積為 V0,壓力為 P0,溫度為 T0, 斷熱壓縮後之體積為 V,壓力為 P,溫度為 T,若 CP為定壓比熱(molar specific heat at constant pressure),CV為定容比熱(molar specific heat at constant volume),且 r = CP/CV,則其間的關係 式,下列何者正確?
(A) P/P0 = (V0/V) r
(B) (T/T0) r = P0/P
(C) (P0/P)r = T0/T
(D) T0/T = (V0/V)1-r
答案:登入後查看
統計: A(296), B(136), C(163), D(128), E(0) #3214102
統計: A(296), B(136), C(163), D(128), E(0) #3214102