題組內容
2. 1.75 mole of an ideal gas with 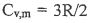 (heat capacity per mole at
constant volume) is expanded adiabatically against a const external pressure of 1 bar.
The initial temperature and pressure are 290 K and 19.5 bar. The final pressure is l
bar.
(heat capacity per mole at
constant volume) is expanded adiabatically against a const external pressure of 1 bar.
The initial temperature and pressure are 290 K and 19.5 bar. The final pressure is l
bar.
2. 1.75 mole of an ideal gas with 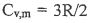 (heat capacity per mole at
constant volume) is expanded adiabatically against a const external pressure of 1 bar.
The initial temperature and pressure are 290 K and 19.5 bar. The final pressure is l
bar.
(heat capacity per mole at
constant volume) is expanded adiabatically against a const external pressure of 1 bar.
The initial temperature and pressure are 290 K and 19.5 bar. The final pressure is l
bar.