題組內容
6. Methane (CH4) is an important greenhouse gas in the atmosphere. Currently, the
average concentration of CH, in the atmosphere is 1.75 ppmv, and it will react with
the hydroxyI radicals (ㆍOH). The reaction formula is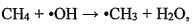 and the reaction rate R = k[CH4] [ ㆍOH]. Where the reaction rate constant k=
and the reaction rate R = k[CH4] [ ㆍOH]. Where the reaction rate constant k=
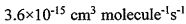 . Assuming that the concentration of hydroxyl radicals
(ㆍOH) in the atmosphere is 8.5 x 105 molecules
. Assuming that the concentration of hydroxyl radicals
(ㆍOH) in the atmosphere is 8.5 x 105 molecules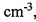 , and 1.0 ppm CH4 = 1.0 mole
CH4/106 mole air.
, and 1.0 ppm CH4 = 1.0 mole
CH4/106 mole air.