題組內容
2. The bonding in Hydrogen Iodide (HI) can be described by a two orbital interaction between the 1s orbital on Hydrogen and the 5p orbital on lodine. The Hamiltonian matrix clements (coulomb integrals, exchange integral and overlap integral) are: 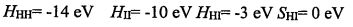
Assume that the molecular wave function is:
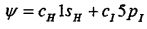
(b) Use your answer in part (a) to calculate the coefficients,  and C1, in the Normlized anti-bonding orbital. If you are not sure of the energy, then use
and C1, in the Normlized anti-bonding orbital. If you are not sure of the energy, then use  = -8.0 eV to work out this part.
= -8.0 eV to work out this part.