題組內容
8.We consider the irreversible freezing of water below its freezing point. The freezing of a mole of water at -10°C is an irreversible change, but can be carried out reversibly by means of the three steps.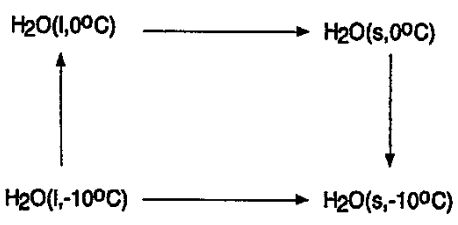
(c) (5%) What is the Gibbs free energy of freezing water at -10°C? (Molar melting enthalpy of ice: △mH° = 5619 J mol -1at–10°C, △mH° = 6008 J mol-¹ at 0°C; molar heat capacity: ice Cp,m=37.66 J mol-¹K-¹, water Cp,m-75.38 J mol¹K¹)