題組內容
3. Zirconium is one of the few metals that retains its structure integrity upon exposure to radiation. The fuel rods in most nuclear reactors therefore are often made of zirconium. Answer the following questions about the redox properties of zirconium based on the half-reaction:
ZrO2‧H2O + H2O +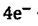 → Zr+
→ Zr+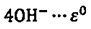 = -2.36 V
= -2.36 V
a. Use the appended standard reduction half potential, is zirconium metal capable of reducing water to form hydrogen gas at standard conditions?
2H2O+2e → H2 +  = -0.83 V
= -0.83 V