16. For the Arrhenius equation, k = exp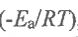 , which of the following statement is CORRECT?
, which of the following statement is CORRECT?
(A) The effect of an enzyme is to increase the magnitude of A.
(B) The exp (-Ea/RT) term represents the fraction of molecules with a kinetic energy larger than Ea.
(C) The reaction rate k is linearly proportional to temperature T.
(D) The activation energy Ea is associated with the collision frequency of the reactants.
(E) The steric factor of reaction affects the magnitude of Ea.
答案:登入後查看
統計: A(0), B(1), C(0), D(2), E(0) #3421211
統計: A(0), B(1), C(0), D(2), E(0) #3421211