20. You have 500.0 mL of a buffer solution containing 0.20 M acetic acid (CH3COOH) and 0.30 M sodium acetate (CH3COONa). What will the pH of this solution be after the addition of 20.0 mL of 1.00 M NaOH solution?
(A) 4.41
(B) 4.74
(C)4.56
(D) 4.92
(E) 5.07
答案:登入後查看
統計: A(0), B(4), C(0), D(4), E(5) #2973574
統計: A(0), B(4), C(0), D(4), E(5) #2973574
詳解 (共 3 筆)
#6361278
(1)Henderson-Hasselbalch方程式,計算初始的pKa值
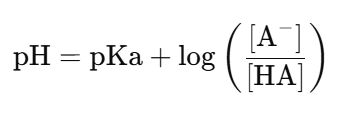
ㅤㅤ
(2)加入20.0 mL 1.00 M NaOH後,NaOH會中和一部分的醋酸
0.02 mole的NaOH會將0.02 mole的CH₃COOH轉為CH₃COO⁻,造成:
-
[HA] 的濃度減少 0.02 mole
-
[A⁻] 的濃度增加 0.02 mole
新的 [HA] 濃度:[(0.20×0.500)−0.02]/0.52=0.1538M
新的 [A⁻] 濃度:[(0.30×0.500)+0.02]/0.52=0.3269M
ㅤㅤ
(3)再用Henderson-Hasselbalch方程式,計算pH值
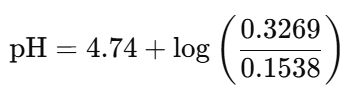
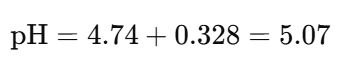
1
0