22. For the Arrhenius equation, k = A exp(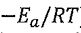 ), which of the following statement is wrong?
), which of the following statement is wrong?
(A). The effect of catalyst is to increase the magnitude of A.
(B). The pre-exponential factor A is associated with the collision frequency of reactants.
(C). The activation energy 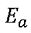 can be positive or negative.
can be positive or negative.
(D). The pre-exponential factor A includes the steric factor of reaction.
(E). exp(-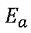 /RT) represents the fraction of molecules with a kinetic energy larger than Ea.
/RT) represents the fraction of molecules with a kinetic energy larger than Ea.
答案:登入後查看
統計: 尚無統計資料
統計: 尚無統計資料