55. Which of the following statements is false for one mole of monatomic ideal gas?
(A) By the Equivalence Theorem, we know that the average energy of each molecule in a monatomic gas is 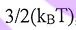 , where the translation, rotational and vibrational motion each contribute equally to the energy
, where the translation, rotational and vibrational motion each contribute equally to the energy 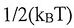 .
.
(B) The isobaric process requires absorbing more heat to increase its temperature by one unit than the isochoric process.
(C) If the pressure of a gas is doubled and the volume is halved, the internal energy of the gas does not change.
(D) During a reversible adiabatic process, the ideal gas law PV = nRT still holds.
(E) None of the above is false.
答案:登入後查看
統計: A(2), B(0), C(0), D(0), E(0) #3422526
統計: A(2), B(0), C(0), D(0), E(0) #3422526