6.Consider the following ground state electron configuration: 1s22s22p4. Which of the
ions has this ground state electron configuration?
(A)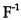
(B)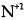
(C)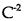
(D)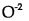
答案:登入後查看
統計: A(0), B(0), C(9), D(0), E(0) #2740017
統計: A(0), B(0), C(9), D(0), E(0) #2740017