題組內容
2.已知下列各化學反應式:
2NH3(g) + 3N2O(g) → 4N2(g) + 3H2O(l) △H= -241.4 kcal
N2O(g) + 3H2(g) → N2H4(l) + H2O(l) △H= -75.7 kcal
2NH3(g) +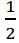 O2(g) → N2H4(l) + H2O(l) △H= -34.2 kcal
O2(g) → N2H4(l) + H2O(l) △H= -34.2 kcal
H2(g) + 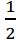 O2(g) → H2O(l) △H= -68.3 kcal
O2(g) → H2O(l) △H= -68.3 kcal
2.已知下列各化學反應式:
2NH3(g) + 3N2O(g) → 4N2(g) + 3H2O(l) △H= -241.4 kcal
N2O(g) + 3H2(g) → N2H4(l) + H2O(l) △H= -75.7 kcal
2NH3(g) +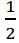 O2(g) → N2H4(l) + H2O(l) △H= -34.2 kcal
O2(g) → N2H4(l) + H2O(l) △H= -34.2 kcal
H2(g) + 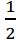 O2(g) → H2O(l) △H= -68.3 kcal
O2(g) → H2O(l) △H= -68.3 kcal