題組內容
3. For a closed simple compressible system operating with air as an ideal gas with molecular weight M as well
as constant specific heats cp and cv where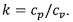 . The temperature T, pressure p, specific volume v
and specific entropy s of two equilibrium states of this system are given as (T1, p1, v1, S1) and (T2, p2, v2, S2),
respectively. The universal gas constant is
. The temperature T, pressure p, specific volume v
and specific entropy s of two equilibrium states of this system are given as (T1, p1, v1, S1) and (T2, p2, v2, S2),
respectively. The universal gas constant is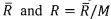 . Please derive the following relations. (32%)
. Please derive the following relations. (32%)
(d) The thermal eficiency  of a cold air-standard Otto cycle with the compression ratio r where its processes are modeled by a closed simple compressible system.
of a cold air-standard Otto cycle with the compression ratio r where its processes are modeled by a closed simple compressible system.