題組內容
8.We consider the irreversible freezing of water below its freezing point. The freezing of a mole of water at -10°C is an irreversible change, but can be carried out reversibly by means of the three steps.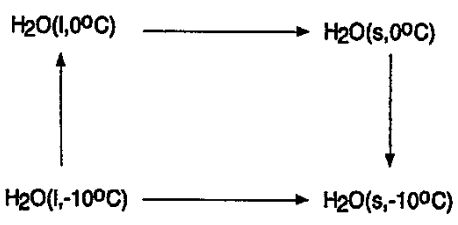
(a) (5%) Calculate the change in the entropy when 1 mol of liquid water at -10°C changes to ice at -10°C. Explain your result!