載入中..請稍候..
【詳解卡福利】寫作批改懸賞券,將於 2025/04/30 23:59:59 過期,還沒使用或領取,趕快前往領取並使用吧! 前往查看
1. One mole of N2(g) at 298 K expand..
1. One mole of N2(g) at 298 K expands adiabatically from a volume of 5.0 atm to 1.0 atm in following two conditions: (a) reversibly, and (b) against a constant external pressure of 1.0 atm. The molar heat capacity at constant volume is equal to 5R/2. Determine the values of final temperature of N2(g), for conditions (a) and (b). R = 8.314 J 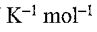 = 0.082 L atm
= 0.082 L atm 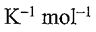
This is a large modal.