載入中..請稍候..
3. Zirconium is one of the few metals that retains its structure integrity upon exposure to radiation. The fuel rods in most nuclear reactors therefore are often made of zirconium. Answer the following questions about the redox properties of zirconium based on the half-reaction:
ZrO2‧H2O + H2O +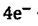 → Zr+
→ Zr+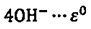 = -2.36 V
= -2.36 V
d. If 1.00 x 103 kg of Zr (91.22 a.u.) reacts, what mass of H2 is produced? What volume of H2 at 1.0 atm and 1000°C is produced?
c. Calculate AG°, and equilibrium constant for the reduction of water by zirconium metal.
b. Write down a balanced equation for the reduction of water by zirconium.
18. The mass percent of carbon in a typical human is 18%, and the mass percent of 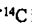 in natural carbon is 1.6 ×
in natural carbon is 1.6 × 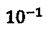 %. For an 80-kg person, how many decay events per second occur in this person due to the p-particle decay of
%. For an 80-kg person, how many decay events per second occur in this person due to the p-particle decay of 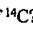 ? (For
? (For 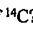 , half-life for ẞ-decay is 5730 years.) (A) 2800 (B) 3800 (C) 4800 (D) 5800
, half-life for ẞ-decay is 5730 years.) (A) 2800 (B) 3800 (C) 4800 (D) 5800
17. Which of the following statement is true?. (A) The coordination number of a metal ion in an octahedral complex is 8. (B) All tetrahedral complex ions are low-spin. (C) The formula for triaquatriamminechromium(III) sulfate is [Cr(H2O)3(NH3)3]2(SO4)3. (D) Hemoglobin contains Fe3+.
16. Concerning the energy split of orbital in octahedral 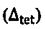 and tetrahedral
and tetrahedral 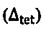 crystal field, which of the following relationship between
crystal field, which of the following relationship between 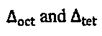 is true? (A)
is true? (A) 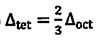 (B)
(B) 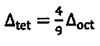 (C)
(C) 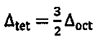 (D)
(D) 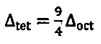
This is a large modal.