活動名稱
【解題達人! We want you!】
活動說明
阿摩站上可謂臥虎藏龍,阿摩發出200萬顆鑽石號召達人們來解題!
針對一些題目可能有疑問但卻缺少討論,阿摩主動幫大家尋找最佳解!
懸賞試題多達20萬題,快看看是否有自己拿手的科目試題,一旦你的回應被選為最佳解,一題即可獲得10顆鑽石。
懸賞時間結束後,只要摩友觀看你的詳解,每次也會得到10顆鑽石喔!
關於鑽石
如何使用:
- ✔懸賞試題詳解
- ✔購買私人筆記
- ✔購買懸賞詳解
- ✔兌換VIP
(1000顆鑽石可換30天VIP) - ✔兌換現金
(50000顆鑽石可換NT$4,000)
如何獲得:
- ✔解答懸賞題目並被選為最佳解
- ✔撰寫私人筆記販售
- ✔撰寫詳解販售(必須超過10讚)
- ✔直接購買 (至站內商城選購)
** 所有鑽石收入,都會有10%的手續費用
近期考題
【非選題】
【題組】
2. One mole of a gas is placed in a closed system with a 0.02 m3 vessel initially at T = 300 K.
The vessel is then isothermally expanded to 0.04 m3. The gas follows the equation of state: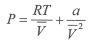
where a=-4.053 m6 Palmol2 and R=8.314 J/mol K.
【題組】
(2) Find for the gas in this process. (10%)
for the gas in this process. (10%)
<Hint> Expansion rule:
5.以下何種激勵方案,對於提升生產製造及業務單位的團隊績效最有幫助?
(A)現金利潤分享計畫(cash profit sharing plans)
(B)資本增益計畫(assetaccumulation plans
(C)盈餘分享計畫(gain-sharing plans
(D)遞延利潤分享計畫(deferredprofit-haring plans
(E)員工持股計畫(employeestock ownership plans)
(A)現金利潤分享計畫(cash profit sharing plans)
(B)資本增益計畫(assetaccumulation plans
(C)盈餘分享計畫(gain-sharing plans
(D)遞延利潤分享計畫(deferredprofit-haring plans
(E)員工持股計畫(employeestock ownership plans)
29.依產業園區土地建築物與設施使用收益及處分辦法規定,有關產業園區內土地或房屋,如因遺漏徵收或調整開發範
圍而補辦徵收時之處理方式為何?
(A)唯一途徑只能領現金補償
(B)只有土地所有人能再辦理配售
(C)由原辦理配售機關按被徵收所有權人持有之權利價值,就產業園區內尚未配售之社區用地,依該辦法第 40 條規定辦理 配售
(D)由原辦理配售機關按被徵收所有權人持有之權利價值,就產業園區內社區用地,依該辦法第 45 條規定辦理配售
(A)唯一途徑只能領現金補償
(B)只有土地所有人能再辦理配售
(C)由原辦理配售機關按被徵收所有權人持有之權利價值,就產業園區內尚未配售之社區用地,依該辦法第 40 條規定辦理 配售
(D)由原辦理配售機關按被徵收所有權人持有之權利價值,就產業園區內社區用地,依該辦法第 45 條規定辦理配售
【非選題】
二、在民國108年1月9日公布的【國家語言發展法】中,要求國家語言應為 國民基本教育各階段之部定課程,並預計於民國111年開始實施。、請說明 國家語言發展法的主要理念二、如果教育部決定以每周一節課的方式進 行,你可以接受此一做法嗎?請說明理由。
二、在民國108年1月9日公布的【國家語言發展法】中,要求國家語言應為 國民基本教育各階段之部定課程,並預計於民國111年開始實施。、請說明 國家語言發展法的主要理念二、如果教育部決定以每周一節課的方式進 行,你可以接受此一做法嗎?請說明理由。