

活動名稱
【解題達人! We want you!】
活動說明
阿摩站上可謂臥虎藏龍,阿摩發出200萬顆鑽石號召達人們來解題!
針對一些題目可能有疑問但卻缺少討論,阿摩主動幫大家尋找最佳解!
懸賞試題多達20萬題,快看看是否有自己拿手的科目試題,一旦你的回應被選為最佳解,一題即可獲得10顆鑽石。
懸賞時間結束後,只要摩友觀看你的詳解,每次也會得到10顆鑽石喔!
關於鑽石
如何使用:
- ✔懸賞試題詳解
- ✔購買私人筆記
- ✔購買懸賞詳解
- ✔兌換VIP
(1000顆鑽石可換30天VIP) - ✔兌換現金
(50000顆鑽石可換NT$4,000)
如何獲得:
- ✔解答懸賞題目並被選為最佳解
- ✔撰寫私人筆記販售
- ✔撰寫詳解販售(必須超過10讚)
- ✔直接購買 (至站內商城選購)
** 所有鑽石收入,都會有10%的手續費用
近期考題
【非選題】
【題組】⑶說明颱風的強度如何影響其加強速率?並舉例。(5 分)
五、已知在氣壓圓柱坐標(r, θ, p)內,準軸對稱颱風渦旋之切向運動方程如下: 其中,Vr、Vθ、Fθ 分別為徑向速度、切向速度及切向摩擦加速度,其餘符號為一般
常用者,上方橫線則表示沿方位角之軸向平均。試回答下列問題:
其中,Vr、Vθ、Fθ 分別為徑向速度、切向速度及切向摩擦加速度,其餘符號為一般
常用者,上方橫線則表示沿方位角之軸向平均。試回答下列問題:
【題組】⑶說明颱風的強度如何影響其加強速率?並舉例。(5 分)
12.甲公司2008年3月在上年度财务会计报告批准报出前发现一台管理用固定资产未计提折旧,属于重大差错。该固定资产系2006年6月接受乙公司捐赠取得。根据甲公司的折旧政策,该固定资产2006年应计提折旧100万元,2007年应计提折旧200万元。假定甲公司按净利润的10%提取法定盈余公积,不考虑所得税等其他因素,甲公司2007年度资产负债表"未分配利润"项目"年末数"应调减的金额为()万元
(A)90
(B)180
(C)200
(D)270
(A)90
(B)180
(C)200
(D)270
【非選題】
三、請根據我國人口販運防制法(中華民國 98 年 1 月 23 日公布施行)之相關條文規定, 論述現行我國對於被販運之非本國人或無國籍之大陸、港澳地區人民相關之通報、 救援、安置及保護輔導相關措施,並分析當前所遭遇之問題。(25 分)
三、請根據我國人口販運防制法(中華民國 98 年 1 月 23 日公布施行)之相關條文規定, 論述現行我國對於被販運之非本國人或無國籍之大陸、港澳地區人民相關之通報、 救援、安置及保護輔導相關措施,並分析當前所遭遇之問題。(25 分)
【非選題】
【題組】
3. For a closed simple compressible system operating with air as an ideal gas with molecular weight M as well
as constant specific heats cp and cv where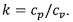 . The temperature T, pressure p, specific volume v
and specific entropy s of two equilibrium states of this system are given as (T1, p1, v1, S1) and (T2, p2, v2, S2),
respectively. The universal gas constant is
. The temperature T, pressure p, specific volume v
and specific entropy s of two equilibrium states of this system are given as (T1, p1, v1, S1) and (T2, p2, v2, S2),
respectively. The universal gas constant is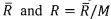 . Please derive the following relations. (32%)
. Please derive the following relations. (32%)
【題組】
(c) For an isentropic process,