9. 請以提供的英文科學文章:
(1)撰寫 100-300 字數的中文科學素養題的文本。(8 分)
(2)根據此文本,出 2 題科學素養題,題型為五選項的多重選擇題,並製作試題的詳細解析。(5 分)
Adenylate cyclase activity of TIR1/AFB links cAMP to auxin-dependent responses The phytohormone auxin is essential for plant growth and development as well as cellular and systemic responses to environmental cues. The auxin receptor TRANSPORT INHIBITOR RESPONSE 1/AUXIN-SIGNALING F-BOX (TIR1/AFB) lies within an E3 ubiquitin ligase complex (SCFTIR1/AFB) consisting of Skp, Cullin, and F-box proteins. Auxin brings together TIR1/AFB and the transcriptional repressor Auxin/INDOLE-3-ACETIC ACID (Aux/IAA), which allows the SCFTIR1/AFB complex to transfer activated ubiquitin to Aux/IAA, thereby targeting it for proteasomal degradation. This liberates the transcription factors AUXIN-RESPONSE FACTORs for auxin-dependent transcription. Just when we thought that the major components in the auxin signaling pathway had been identified, an adenylate cyclase (AC), embedded in the TIR1/AFB receptors, has emerged as an essential component of auxin signaling in gravitropism and root growth inhibition (Figure 1). The product of the AC, 3′,5′-cyclic adenosine monophosphate (cAMP) could also mediate effects of auxin, especially in fastgrowing cells at the apical meristems or in polarized tip growth of pollen tubes, plausibly via the modulation of Ca2+ and changes in the actin cytoskeleton.
Through sequence analysis of Arabidopsis thaliana TIR1/AFBs, Jiri Friml’s group has identified an AC amino acid motif at the C-terminal that is relatively conserved and confers similar levels of catalytic activity as other plant ACs that harbor the same AC motif. The cAMP generated by AFB1, AFB5, and TIR1 was assessed with tandem liquid chromatography mass spectrometry. A phylogenetic analysis indicated that the AC motif is also present, conferring AC activity, in all four orthologs from the moss Physcomitrella patens, thus implying that this moonlighting AC in TIR1/AFB is of ancient origin. To probe the function of key amino acids in the AC motif [RKS]X[DE]X(9,11)[KR]X(1,3)[DE], Qi et al. 2022) generated three mutant variants of AFB5 replacing the residues in positions 3 [DE], 15 [R], and 17 [D] with alanine, respectively. These three mutant variants had weak or no activity in vitro and failed to rescue the AC-deficient E. coli SP850. However, pull-down experiments with IAA showed that only the variant with a substituted [R] at position 15 of the motif abolished the interaction between TIR1 and IAA7. The AC activity of the other two mutant variants does not affect auxin-induced interactions of TIR1 with Aux/IAA (Figure 1A).
Furthermore, structural analysis of the TIR1–IAA–Aux/IAA complex showed that the auxin-binding pocket is situated close to the AC, and computational simulation confirmed that ATP could dock at the AC center, assuming a binding pose that is consistent with that reported in other plant ACs harboring this motif. While not obstructing substrate binding, ATP movement might be restricted by a valine residue from Aux/IAA, thus conceivably increasing the efficiency of the AC. This was indeed observed in recombinant ABF5 and TIR1 where their AC activities were increased in the presence of both IAA and IAA7 or IAA17 and not with IAA or IAA7/17 alone, thus implying that the auxin-induced TIR1–Aux/IAA complex enhances the activity of the AC. In roots of Col-0 Arabidopsis seedlings, increases in cAMP levels were reproducibly detected from 1 h after IAA treatment, and this was not observed in the tir triple mutant (tir1-1 afb2-1 afb3-1).
The authors further demonstrated the effect of AC mutations in planta using the tir1-1 afb2-3 double mutant, which is resistant to auxin-induced root growth inhibition. While the introduction of pTIR1::TIR1 could complement the mutant phenotype, all three mutant variants showed reduced complementation effects. The variant with a substituted [R] at position 15 of the AC motif was least responsive to IAA, and this could be due to its inability to interact with Aux/IAA. Moreover, restoration of the gravitropic root bending in tir1-1 afb2-3 is also reduced in the variants harboring a mutation at positions 3 and 17 of the AC motif (Figure 1B).
The authors also generated pTIR1::ccvTIR1 and the corresponding mutant variants and transformed them into tir1-1 afb2-3. ccvTIR1 contains an engineered auxin-binding site that only accommodates, and is activated by, cvxIAA, but not IAA. Conversely, cvxIAA also does not bind or activate the natural TIR1/AFB. As expected, cvxIAA only triggers root growth
inhibition in ccvTIR1 transgenic plants. Importantly, ccvTIR1 harboring a mutation at position 15 of the AC motif was completely resistant to cvxIAA-dependent root growth inhibition, whereas other mutant variants showed slight root growth inhibition. This convincingly links the AC activity in TIR to auxin-dependent root growth inhibition and gravitropism (Figure 1B).
What started as a quest to identify the molecular mechanism of rapid auxin-dependent root responses led to the discovery of a novel component of the nuclear auxin pathway. It was shown that the AC activity of TIR1/AFB is indeed not required for the rapid auxin-dependent root responses). pTIR1:ccvTIR1 and the variant harboring a mutation at position 3 of the AC motif showed no significant difference in root growth inhibition within the first hour of cvxIAA treatment. This mutant variant only showed resistance to cvxIAA after 1 h of treatment, which corresponded with the dynamics of cAMP levels in root tissues. Previous studies have shown that this rapid auxin-induced root growth inhibition is linked to elevation of cytosolic Ca2+ and apoplast alkalinization, neither of which were altered in this mutant variant compared with ccvTIR1. In contrast, the cvxIAAinduced activation of GH3.3, GH3.5, IAA5, IAA19, and LBD29 transcription in ccvTIR1 plants was reduced in this mutant line, thus affirming the role of the AC in the slower canonical auxin pathway (Figure 1A). Since the authors demonstrated that the AC in TIR1/AFB is not required for the non-transcriptional auxin responses, the molecular mechanisms underpinning the rapid response to auxin in the root have remained elusive .
The findings of Qi et al. (2022) also invite speculations on the identity of the cAMP degrading enzymes, the phosphodiesterases (PDEs), which have been recently identified in monocot and dicot plants using a similar motif search approach. Interestingly, the Arabidopsis thaliana K+ -uptake permease 5 (AtKUP5), the Brassica napus Bifunctional protein FolD 2, and the Marchantia polymorpha COMBINED AC with PDE (MpCAPE), all harbor both AC and PDE domains, thus qualifying them as molecular switches that could fine-tune localized cAMP levels. This feature conceivably makes them attractive targets for crop improvements.
In summary, the findings of Qi et al. (2022) therefore constitute a breakthrough in plant cyclic nucleotide research, which was, in the past, the subject of controversy surrounding the cryptic nature of the generating enzymes and was hampered by a lack of significant in planta evidence demonstrating a biological role of plant ACs. The results of Qi et al. (2022), together with an increasing number of plant ACs discovered in recent years, and detailed descriptions of AC in complex molecules such as ion channels are now dispelling some persisting doubts about cyclic nucleotide signaling in plants. Taken together, these findings will guide the search for intra- and inter-molecular mechanisms enabled by moonlighting ACs in other complex multi-domain proteins. Once their downstream effectors become more clearly defined, ACs and cAMP will, much like in animals and bacteria, be finally established as major signalling components in plants.
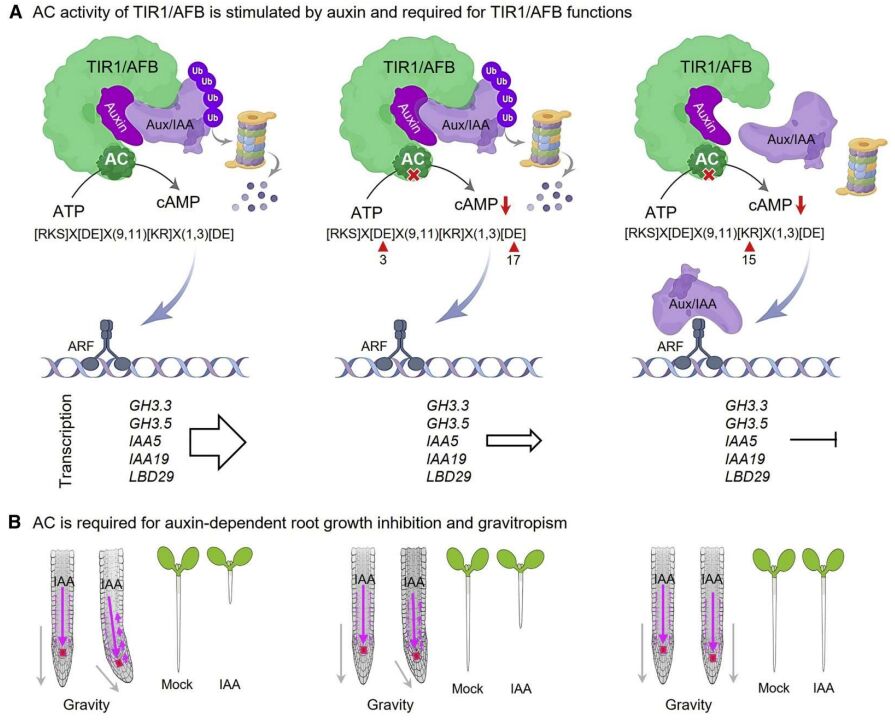
Figure 1Adenylate cyclase auxin-dependent effects of TIR1/AFB.
(A) The AC activity of TIR1/AFB is stimulated by auxin and is required for auxin-dependent transcription. Substitution of amino acid at positions 3 and 17 of the AC motif inactivates the AC. The mutation of the amino acid at position 15 reduces the AC activity and interferes with the formation of the TIR1/AFB–Aux/IAA complex, which is essential for the lifting of repression on the ARF transcription factors, achieved through the proteasomal degradation of SCFTIR1/AFB ubiquitinated Aux/IAA. The AC motif with a mutation at position 3 reduces auxin-dependent transcription.
(B) The AC activity of TIR1/AFB is required for auxin-dependent root growth inhibition and gravitropism. Substitution of amino acid at positions 3 and 17 of the AC motif reduces the auxin-dependent root growth inhibition and gravitropism, while mutation of amino acid at position 15 completely desensitizes the root to auxin.