27.The reaction: 2 HI→ H2 + I2, is second order and the rate constant at 800 K is 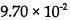
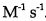 . How long will it take for 8.00 x
. How long will it take for 8.00 x 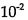 mol/L of HI to decrease to one-fourth of its initial concentration?
mol/L of HI to decrease to one-fourth of its initial concentration?
(A) 0.619s
(B) 124 s
(C) 387s
(D) 429 s
答案:登入後查看
統計: A(1), B(2), C(6), D(1), E(0) #2740038
統計: A(1), B(2), C(6), D(1), E(0) #2740038