題組內容
13. l atm、30℃下,空氣中含水蒸氣的分壓為 20mmHg。若30℃時,飽和水蒸氣壓為32mmHg,
請求出空氣的:(空氣的平均分子量為 29 g/mol)
(3)百分濕度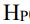 (2分)
(2分)
詳解 (共 1 筆)
詳解
l atm、30℃下,空氣中含水蒸氣的分壓為 20mmHg。若30℃時,飽和水蒸氣壓為32mmHg, 請求出空氣的:(空氣的平均分子量為 29 g/mol)
百分濕度
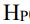
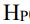
公式1
Hp = H / Hs *100%
= 0.0168 /0.027 *100%
= 61.58%
公式2
Hp = PH2O (Pt - PoH2O ) / PoH2O (Pt - PH2O )
= 20 (760-32) /[32 (760-20)]
= 0.6149
公式3
Hp = HR * (Pt - PoH2O ) / (Pt - PH2O )
= 62.5 % * (760-32)/(760-20)
=0.6149