題組內容
23. After constructing energy-level diagrams of molecular orbital (MO) for six homonuclear diatomic molecules: B2, C2, N2, O2, F2, Ne2. Please answer the following questions.
(Note that the electron configuration of Ne2 is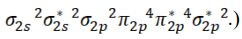
(3) In B2, the energy level of i σ2p s higher compared to that of π2p , whereas in O2, it is the reverse, with the energy of σ2pbeing lower than that ofπ2p. Elucidate this phenomenon. (1 point)