題組內容
12. A solution is made by adding some CuSO4•5H2O to water, followed by enough sulfuric acid to make the pH = 1.00. Two platinum electrodes are then placed in the solution (which has a total volume of 0.500 L) and the solution is electrolyzed at a constant current of 0.120 A, separately capturing any gases that are evolved at the two electrodes.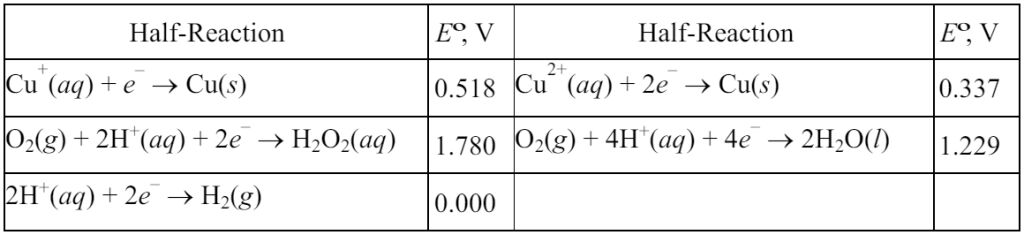
Initially, gas is evolved at the anode, but no gas is evolved at the cathode. However, after 10.0 min of electrolysis, gas evolution begins at the cathode as well, and eventually the total volume of gas evolved at the cathode is equal to the total volume of gas evolved at the anode.
