載入中..請稍候..
以下考題(第26到第30題)請由下表27個答案選項中選出一個正確答案,並將答案代碼填入答案卡。例:若答案為D4h,請於該題答案卡上畫上代碼(B)(C)(D)。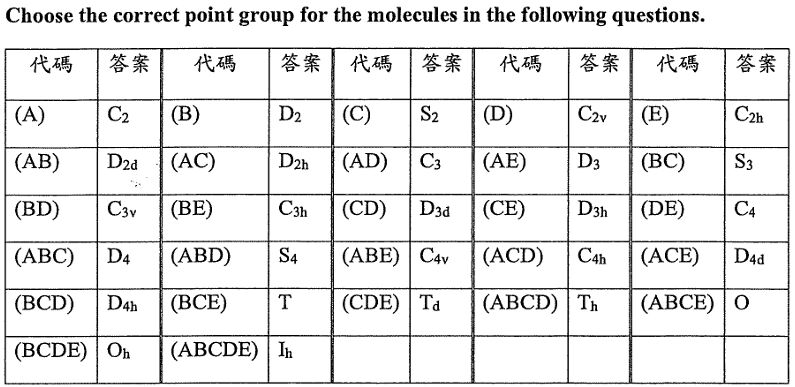
30. Ni(cyclobutadiene)2 staggered
29. W[N(CH3)2]6 (ignore the H atoms of the methyl groups)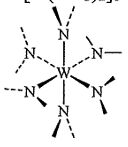
28. 1,3,5,7-tetrafluorocyclooctatetraene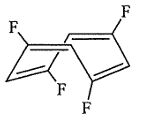
25. Which of the following statements accurately describe aspects of Tanabe-Sugano diagrams for octahedral transition metal complexes? (A) They plot the energy of electronic states relative to the Racah parameter B (B) They apply only to high-spin d-electron configurations. (C) They can illustrate spin-allowed and spin-forbidden transitions. (D) They are primarily used for predicting magnetic moments. (E) They provide information on ligand field splitting as a function of the ligand strength.
24. Which of the following statements about ligand binding and reactivity in organometallic complexes are CORRECT? Select all that apply. (A) Strong -acceptor ligands, such as CO, stabilize low-valent metal centers by withdrawing electron density from the metal. (B) Ligand exchange in organometallic complexes often depends on the relative binding affinities of incoming and outgoing ligands. (C) In the migratory insertion of CO into a metal-alkyl bond, the metal-carbon bond is formally cleaved during the reaction. (D) Chelating ligands enhance the thermodynamic stability of a metal complex by increasing the chelate effect. (E) ẞ-Hydride elimination requires a vacant coordination site on the metal to accommodate the hydride.
23. Which of the following statements are correct regarding the magnetochemical series and its role in predicting magnetic behavior in transition metal complexes? (A). The magnetochemical series ranks ligands by their magnetic influence on metal ions, which can help predict high-spin or low-spin states in transition metal complexes. (B) Ligands higher in the magnetochemical series always produce stronger ligand fields, which leads to high-spin states. (C) The magnetochemical series is primarily used to determine electronic transitions in metal-ligand complexes. (D) Strong-field ligands in the magnetochemical series tend to produce low-spin complexes with smaller magnetic moments. (E) The magnetochemical series does not apply to determining spin states in metal-ligand complexes.
This is a large modal.