23. Carbon monoxide is toxic because it binds much more strongly to the iron in hemoglobin than O2 does. The equilibrium constant for the binding of CO is about 200 times that for the binding of O2. That is, for the reactions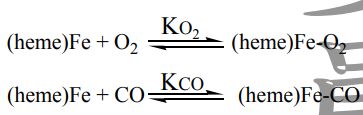
KCO/KO2 = 2.1 ×102. Calculate the difference in ΔG0for the binding of CO and O2 to hemoglobin at 25℃.
(A) –6.5 kJ/mol
(B) –13 kJ/mol
(C) –19.5 kJ/mol
(D) –26 kJ/mol
(E) –39 kJ/mol
答案:登入後查看
統計: 尚無統計資料
統計: 尚無統計資料