34. Normally, heating an initial aldol product (the β-hydroxyaldehyde) causes dehydration. However, heating the compound below with base does not cause dehydration, but rather, decomposition to form 2-ethylbutanal. This is because: 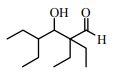
(A) There are no protons β to the carbonyl.
(B) There are no protons adjacent to the OH, and the dehydration reaction requires E1 conditions.
(C) There are no protons α to the carbonyl, and aldol condensations are reversible.
(D) Heating any organic compound above room temperature causes decomposition.
(E) None of these is true.
答案:登入後查看
統計: 尚無統計資料
統計: 尚無統計資料