35. The molar specific heat at constant volume is 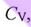 , and the molar specific heat at constant pressure is
, and the molar specific heat at constant pressure is  . For an ideal gas at a fixed temperature, which of the following statements is true?
. For an ideal gas at a fixed temperature, which of the following statements is true?
(A)  is always greater than
is always greater than  , because part of the absorbed heat is used to do work to the environment.
, because part of the absorbed heat is used to do work to the environment.
(B)  is always greater than
is always greater than  , because the environment absorbs additional heat.
, because the environment absorbs additional heat.
(C)  is always less than
is always less than  , because part of the absorbed heat is used to do work to the environment.
, because part of the absorbed heat is used to do work to the environment.
(D) 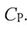 is always less than
is always less than 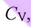 , because the environment absorbs additional heat.
, because the environment absorbs additional heat.
(E) There is no certain relation between  and
and  .
.
答案:登入後查看
統計: A(3), B(2), C(1), D(0), E(0) #3422506
統計: A(3), B(2), C(1), D(0), E(0) #3422506