28. There are four proteins A, B, C, D. (Protein A: homotetramer, 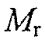 = 280,000, pI =6; Protein B: monomer,
= 280,000, pI =6; Protein B: monomer,  = 113,000, pI=7; Protein C: monomer,
= 113,000, pI=7; Protein C: monomer, 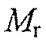 = 13,000, pI=8; Protein D is unknown). When Samuel used a gel filtration column to separate them, the elution sequence is A-D-B-C. Samuel dissolved the protein mixture in 20 mM phosphate buffer (pH 8) and loaded to a anion exchange column equilibrated with the same buffer. Protein D could not bind to this column. On an SDS-PAGE gel, protein D runs faster than protein B and slower than protein C.
= 13,000, pI=8; Protein D is unknown). When Samuel used a gel filtration column to separate them, the elution sequence is A-D-B-C. Samuel dissolved the protein mixture in 20 mM phosphate buffer (pH 8) and loaded to a anion exchange column equilibrated with the same buffer. Protein D could not bind to this column. On an SDS-PAGE gel, protein D runs faster than protein B and slower than protein C.
1. If Samuel used SDS-PAGE to separate A, B, and C. The sequence of the protein bands shown in gel from top to bottom should be A-B-C.
2. The molecular mass of protein D must be between 13 and 113 kDa.
3. The pI of protein D might be higher than 8.
4. D is not a monomer.
5. If Samuel used 2-Dimensional electrophoresis to separate them. We could predict that the relative positions of these four proteins is like the image below.
 (A) 1, 5 are correct. (D) 2, 4 are correct. (B) Only 2 is incorrect. (E) 3, 4 are correct. (C) 1, 3, 4 are correct.
(A) 1, 5 are correct. (D) 2, 4 are correct. (B) Only 2 is incorrect. (E) 3, 4 are correct. (C) 1, 3, 4 are correct.