1.在一單質子弱酸溶液(濃度0.2 M,Ka=2×10-5)中之氫離子濃度為何(M)?
(A)2×10-3
(B)4×10-3
(C)1×10-4
(D)4×10-6
答案:登入後查看
統計: A(2997), B(278), C(451), D(916), E(0) #722548
統計: A(2997), B(278), C(451), D(916), E(0) #722548
詳解 (共 7 筆)
#1421465
1.先看濃度和Ka的比例!(注意陷阱阿~~~)
2.帶入公式

113
1
#1430221
要帶入公式真的會想送他= =
72
8
#1414236
弱酸===> [H+]=√(Ka*C)
弱鹼===>[OH-]=√(Kb*C)
30
2
#2594413
弱酸
ka
HA ↔ A- + H+
(0.2-x) x x
ka= [A-][H+]/[HA]
2×10-5= x2 / (0.2-x)
公式背不起來,我是用以前國中數學概念“配方法”解
2×10-5= x2 / (0.2-x)
x2 − 2×10-5 x +2×10-6 = 0
“配方法”可以得答案
22
0
#6016748
0.2-x的部分
x可省略
所以x2開根號就是答案了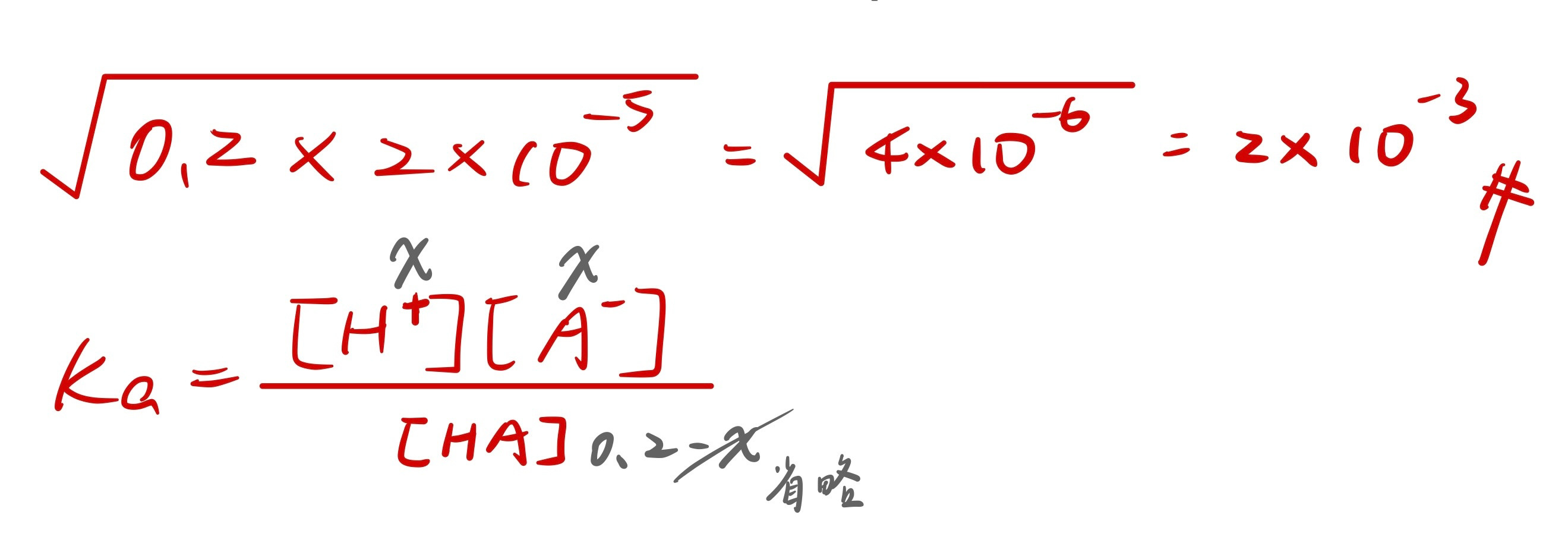
所以x2開根號就是答案了
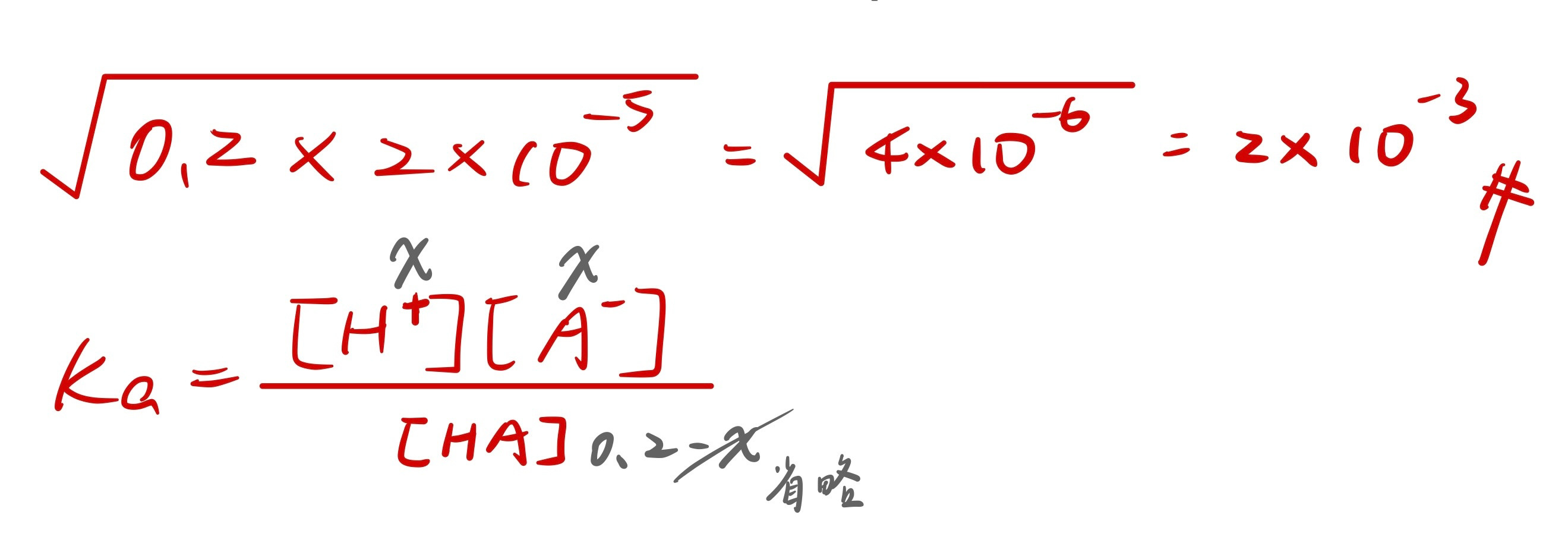
3
0