載入中..請稍候..
2. Consider the base-catalyzed reaction: 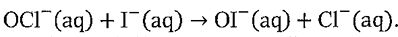 . Use the following data of initial rate v0 to determine (a) the rate law and (b) the corresponding rate constant, including the unit, for the reaction.
. Use the following data of initial rate v0 to determine (a) the rate law and (b) the corresponding rate constant, including the unit, for the reaction.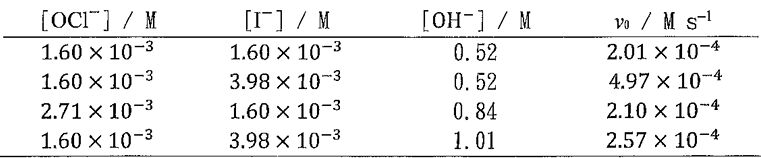
1. One mole of N2(g) at 298 K expands adiabatically from a volume of 5.0 atm to 1.0 atm in following two conditions: (a) reversibly, and (b) against a constant external pressure of 1.0 atm. The molar heat capacity at constant volume is equal to 5R/2. Determine the values of final temperature of N2(g), for conditions (a) and (b). R = 8.314 J 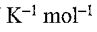 = 0.082 L atm
= 0.082 L atm 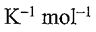
2. The pollinator insects or other animals play the role to accelerate the evolution of angiosperms. The flowers of the earliest members might coevolve with the pollinators, as the angiosperms continued to evolve. Please describe four evolutionary trends of flowers for attracting the pollinators?
2341 為避免工程採購因工期不實導致流標,下列何者非正確因應對策?(A)以日曆天訂定工期,應包含預期可工作日及不可工作日或放假日 (B)檢討工期合理性並於招標文件說明已考量氣候影響之工期天數 (C)細部設計階段確認分項工程、妥適排定施工順序,並考量特殊施工資源,估算各工項工期 (D)僅配合計畫執行期限編列工期。
2340 機關辦理採購應核實編列計畫預算,下列何者非考量之因素?(A)機關需求 (B)使用年限 (C)未來契約可能因廠商違約重行招標之經費 (D)維護管理。
2339 機關應務實評選規劃設計廠商,評定實務可行之方案,評選委員及工作小組應於評選前瞭解之事項包含? (1.計畫需求與預算 2.期程 3.各廠商投標內容 4.契約變更方案)(A)1234 (B)123 (C)124 (D)234。
This is a large modal.